My self Sulekha Rani.R,P.G.T Chemistry,KV INS Dronacharya , Cochin, Kerala .
Search This Blog
Thursday, June 26, 2014
Wednesday, June 25, 2014
COLOURS OF pH INDICATORS
CHEMISTRY BEHIND INDICATORS
Mrs.Sulekha Rani.R , PGT Chemistry, KV NTPC kayamkulam
pH scale actually is is a logarithmic scale for measuring
the concentration of hydrogen ions in a solution. This means that, for every number
you go down on the pH scale, the concentration of hydrogen ions increases by a
factor of ten. The higher the concentration of hydrogen ions in a solution, the
more acidic the solution is.
What does this have to do with the colour changes of
indicator solutions? Well, the indicators themselves are actually either weak
acids or bases. When they’re dissolved in water, their molecules dissociate
slightly and form ions. Indicators tend to be molecules containing a fair
number of alternating (conjugated) carbon-carbon double bonds and single bonds,
such as phenolphthalein, shown below:
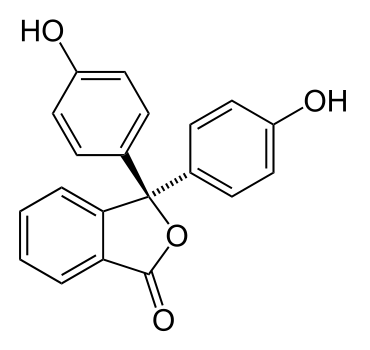
Phenolphthalein
These alternating double/single bonds can absorb wavelengths
from visible light, making them appear coloured. In acidic solutions, the large
number of hydrogen ions already in solution means that the molecule will not
dissociate much, and so the colour seen will be that of the original indicator
molecule. In basic solutions however, the comparative lack of hydrogen ions in
solution leads to the molecule losing a hydrogen ion; this, put simply, changes
the arrangement of electrons in the molecule, causing it to absorb different
wavelengths of light and appear a different colour.
It’s not only set chemical indicators that can be used to
indicate pH changes. Chemicals found naturally in various plants can also be
used – for example, anthocyanin compounds in red cabbage
The Chemistry of the World Cup Football-2014 - BRAZUCA
The Chemistry of the World Cup Football-2014 - BRAZUCA
Mrs . Sulekha Rani.R , PGT Chemistry, KV NTPC kayamkulam

A number of chemical
materials are used in the manufacture of the Brazuca, the World Cup
football. The majority of these materials are polymers; these are very
long molecules built up from many smaller component molecules. A simple,
everyday example is polyethene, used to make some plastic bags.
Different classes of polymers are used to achieve particular properties
for the ball.
Footballs consist of
three main component parts: the covering (the outermost layer), the
lining, and the bladder. Obviously, these will be designed in a manner
that provides the most favourable aerodynamic properties for the ball –
however, that’s veering dangerously into physics territory. None of
these properties would be achievable without chemistry providing the
materials required, so here’s a breakdown of the different types of
polymers used in each component part of the ball.
Covering
The covering of the
ball is made of six polyurethane panels, which are thermally bonded
together. This covering is important to protect the ball, and to prevent
it from absorbing too much water – the water absorption of the Brazuca
ball is just 0.2%. This makes the ball much lighter than the
leather-coated balls used in the past. Some balls may also have a
polyurethane foam layer underneath the covering.
Polyurethanes are built
up from compounds called isocyantes and polyols. The middle parts of
these molecules can be varied to give different polyurethanes with
differing properties. Polyurethanes have a wide range of applications,
including foam in seating, adhesives, synthetic fibres and even
skateboard wheels.
Cheaper footballs may
use PVC (polyvinyl chloride) instead of polyurethane for the coating.
They may also be stitched together, rather than thermally bonded. This
stitching will be made from another class of polymers called polyesters;
on higher end balls this stitching may be reinforced with Kevlar.
Lining
Underneath the covering
layer, the ball will have several layers of lining. These are present
to improve the bounce and strength of the ball. In the World Cup ball,
these are made from another class of polymers, polyamides, more commonly
referred to as nylon. Polyesters can also be utilised for this
purpose.
Nylon and polyesters
are also commonly used components in the manufacture of football shirts,
as well as other clothing. Nylon is additionally used in parachutes,
ropes and fishing nets, whilst polyesters can be found in bed sheets,
carpets and plastic bottles.
Bladder
The bladder is the part
of the football that holds the air. In the Brazuca, this is made from
butyl rubber, but it can also be made from latex. Both have their
benefits: butyl rubber retains the air for a longer period of time,
whilst latex provides better surface tension. Butyl rubber can also be
found in the valve through which air can be pumped into the ball, where
it aids air retention. Silicone valves can also be used.
Most modern chewing gum
also uses food grade butyl rubber to give the gum its elasticity.
Unfortunately, it also contributes the unwanted stickiness of gum. It
can also be found in the inner tubing of tyres.
This is just a peek
into the world of polymers – any plastics you use on a day-to-day basis
are composed from polymers, as well as your clothing, and many other
everyday items. Without synthetic polymers, the World Cup would be
kicking off today with a much more rudimentary ball!
Ref: Compound Interest
Subscribe to:
Comments (Atom)

