CHEMISTRY BEHIND INDICATORS
Mrs.Sulekha Rani.R , PGT Chemistry, KV NTPC kayamkulam
pH scale actually is is a logarithmic scale for measuring
the concentration of hydrogen ions in a solution. This means that, for every number
you go down on the pH scale, the concentration of hydrogen ions increases by a
factor of ten. The higher the concentration of hydrogen ions in a solution, the
more acidic the solution is.
What does this have to do with the colour changes of
indicator solutions? Well, the indicators themselves are actually either weak
acids or bases. When they’re dissolved in water, their molecules dissociate
slightly and form ions. Indicators tend to be molecules containing a fair
number of alternating (conjugated) carbon-carbon double bonds and single bonds,
such as phenolphthalein, shown below:
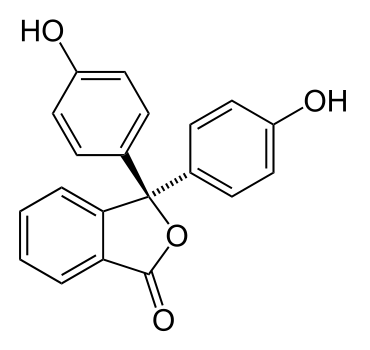
Phenolphthalein
These alternating double/single bonds can absorb wavelengths
from visible light, making them appear coloured. In acidic solutions, the large
number of hydrogen ions already in solution means that the molecule will not
dissociate much, and so the colour seen will be that of the original indicator
molecule. In basic solutions however, the comparative lack of hydrogen ions in
solution leads to the molecule losing a hydrogen ion; this, put simply, changes
the arrangement of electrons in the molecule, causing it to absorb different
wavelengths of light and appear a different colour.
It’s not only set chemical indicators that can be used to
indicate pH changes. Chemicals found naturally in various plants can also be
used – for example, anthocyanin compounds in red cabbage

No comments:
Post a Comment